Do We Want to Be Like Denmark?
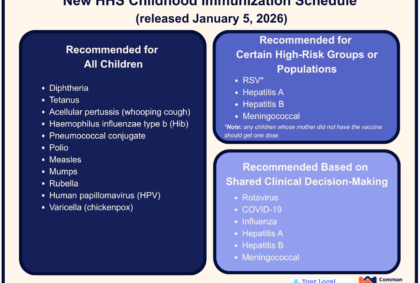
On December 5, 2025, at the insistence of HHS Secretary Robert F. Kennedy, Jr. (a man with no medical degree, or any degree having to do with immunology or vaccinology), President Trump issued an Executive Order asking the US to align ourselves with peer countries that give fewer vaccines. Denmark recommends 10 vaccines, Japan 14, and Germany 15. Do US Children Receive Too Many Vaccines? RFK, Trump, and others believe that too many vaccines are overwhelming, and can weaken immune systems. These individuals frequently point out that, as children, they only received a few vaccines, and they’re fine. And they’re Read More …